Validating Purification Process & Late-Stage Manufacturing
Assay Qualification to Validation
At the early stages of drug development, assay qualification is sufficient, but towards the later stages, validation is necessary to obtain regulatory approval. Like method qualification, method validation is a process that confirms the suitability of a developed assay for its intended analytical use. Results obtained through the validation are used to judge the quality, reliability and consistency of any analytical method. The ICH Q2(R1) guidelines provide guidance on assay validation. Validating an analytical method proves that reliable and reproducible results will be generated with the method, irrespective of time and analyst. Validation must be performed at the laboratory which will routinely perform this method.
At Cygnus Technologies, we have an unmatched and successful industry track record over 25 years of supporting hundreds of biological drug development programs from early process development, to Phase 1-3 clinical manufacturing, and to commercial product lot release:
Expression platforms
Industry gold standard HCP ELISA kits used by most biopharma companies and trusted by global regulatory agencies
HCP antibodies & ELISA kits
Process- and platform-specific kits devloped for many top-tier biopharma companies
Approved cell & gene therapies
24 of 24 approved gene therapies and genetically modified cell therapies use Cygnus HCP analytical technologies for commercial product lot release
Interested in developing a process-specific HCP antibody and assay?
Cygnus’ experts will guide you through the decision process and provide regular updates during the development of your custom assay. At the conclusion of your Process-Specific Ab and Assay Development Project, Cygnus Technologies will provide an “Assay Qualification Report” that will serve as the starting point for your follow-on internal validation or CDMO-outsourced assay validation.
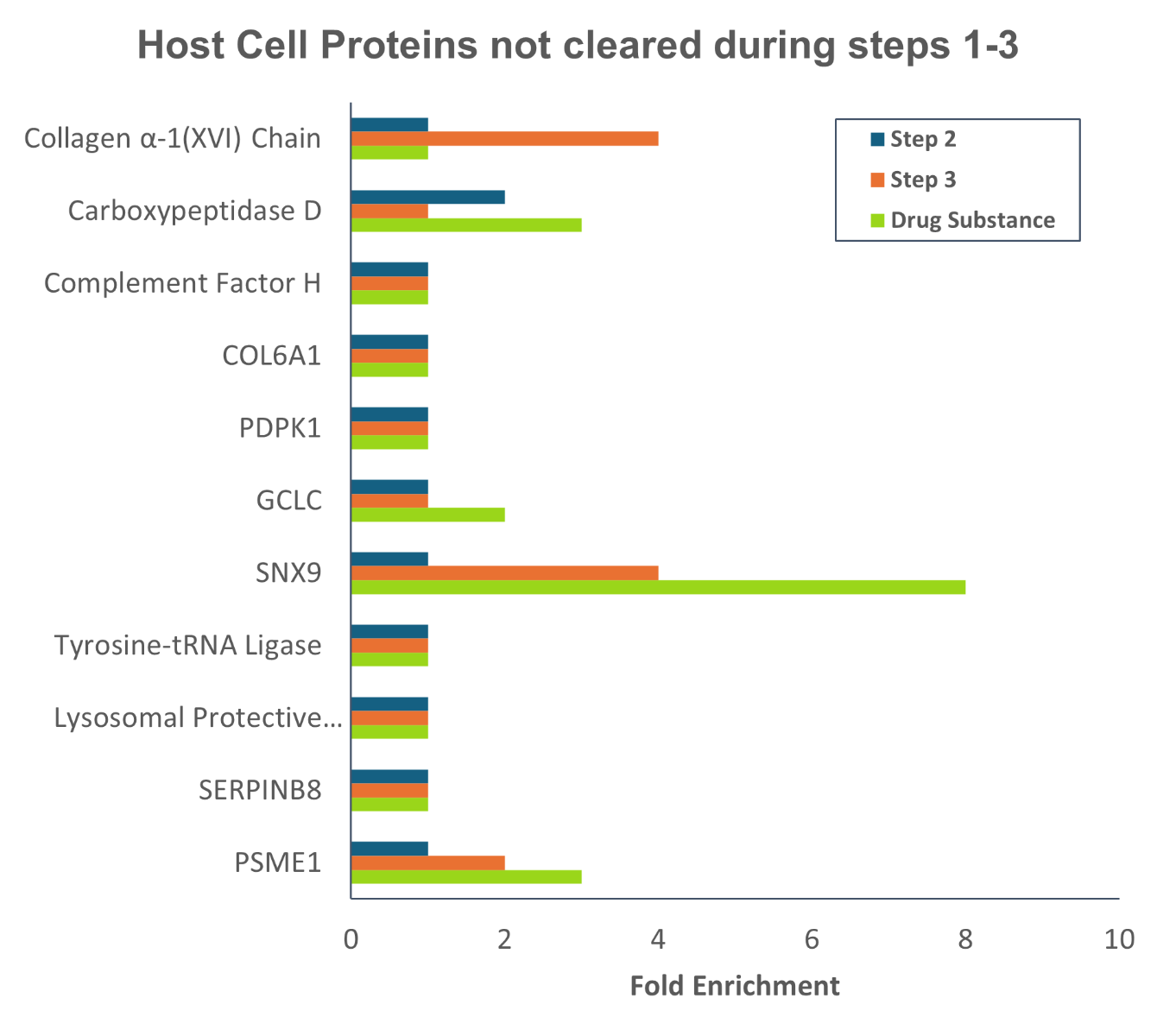
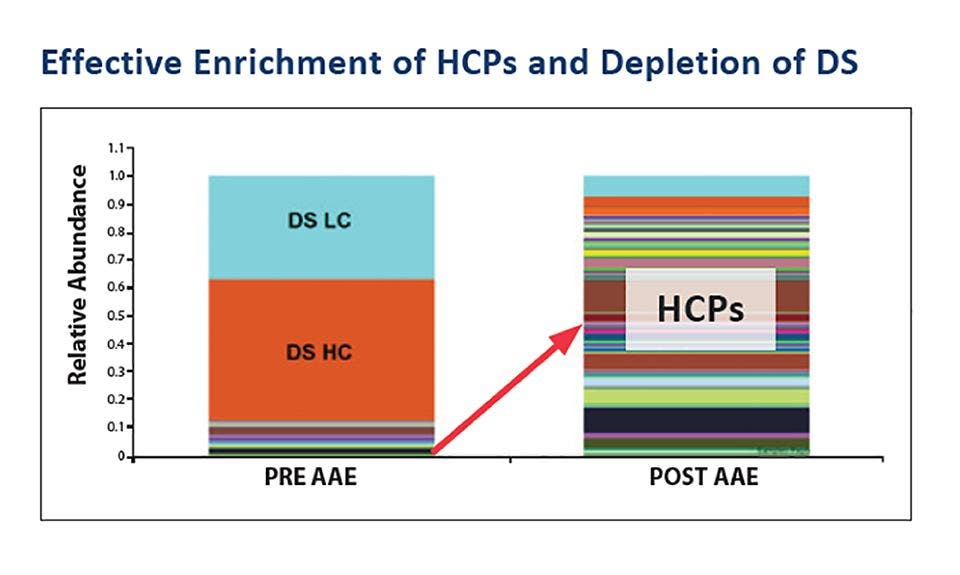
Experiencing HCP enrichment during scale-up or process transfer to a CDMO?
The HCP profile can change during process scale-up, necessitating further adjustments to the downstream purification process. Combining AAE-MS and ELISA methods provides an orthogonal analysis of in-process samples and drug substances to yield a list of specific proteins enriched during process scale up or transfer to another site or CDMO, providing actionable information for process improvements. Get ahead of any potential problems in the clinic by identifying and eliminating problematic host cell proteins that may co-purify with your drug substance.
Generic vs. Process-Specific HCP ELISA
Considering development of a process-specific HCP immunoassay? An HCP assay, regardless of the terminology used to describe it, should be comprehensively qualified to show it is fit for purpose of monitoring host cell protein clearance and lot release testing. Learn about process-specific and generic HCP assays and how to qualify an HCP assay for your specific process.

